What we focus
Daewoong Pharma is engaged in research to contribute to humanity through the development of innovative medicines.

New products
Daewoong Pharma is striving to develop innovative drugs that can improve global health.
We are always welcoming for a new idea in various fields and we can work together, we can work together for global drug development.
-
Indications
Gastroesophageal reflux disease (GERD), gastric and duodenal ulcers, gastritis, etc. (Diseases in relation with gastric acid secretion)
-
Mechanism
Gastric acid secretion is inhibited by reversible proton pump inhibitors (Potassium-competitive acid pump antagonist)
-
Development stage
Phase 1 clinical trial is completed in Korea, and phase 2 clinical trial is underway in the United States, Australia, China, etc.
-
Scheduled date of release
4Q, 2020
-
Partnership areas
Differentiation from PPI products and research to meet unmet medical needs

-
Indication
Neuropathic pain
-
Mechanism
Pain treatment through the inhibition of Nav 1.7 sodium channel
-
Development stage
Identification of Identification of candidate materials
-
Scheduled release date
4Q, 2025
-
Partnership areas
Neuropathic pain clinical indication-related basic research (in vitro, animal models included), Neuropathic pain clinical consultation

-
Indications
Anti-fibrosis (idiopathic pulmonary fibrosis (IPF), cardiac fibrosis, liver fibrosis, and kidney fibrosis)
-
Mechanism
Inhibition of fibrotic factors (α-SMA, Snail, and collagen) synthesis by PRS (Prolyl-tRNASynthetase) enzyme inhibition related to amino acid synthesis
-
Development stage
Identification of Identification of candidate materials
-
Scheduled release date
4Q, 2025
-
Partnership areas
PRS fibrosis-related mode of action, Animal efficacy evaluation system for fibrosis diseases, Assessment model for disease extension such as NASH
-
Indication
Rheumatoid Arthritis
-
Mechanism
Medicines that simultaneously inhibit two targets for autoimmune disease induction
-
Development stage
Identification of candidate materials
-
Scheduled release date
4Q, 2025
-
Partnership areas
Animal models related to immune diseases for expanding indications, Immune cell activation assay, Disease-relevant in vitro assay
-
Indications
Wrinkles, upper limb muscle stiffness, etc.
-
Mechanism
Secretion-blocking of acetylcholine which is a neurotransmitter
-
Development stage
Released in Korea (for wrinkles and upper limb muscle stiffness), Expanding indications (Phase 3 clinical trial - wrinkles, Phase 2 clinical trial – eyelid spasm), Global market (U.S. / Europe: Preparing for product approval, China: Submitted CTA application)
-
Scheduled release date
U.S./Europe in 2018, China in 2022
-
Partnership areas
New indications and animal models, Development of in-vitro toxin activity measuring method to replace animal evaluation, Technology related to the development of recombinant botulinum toxin, Development of products using botulinum toxin other than Type A1.
-
Indications
Type 1 and type 2 diabetes
-
Mechanism
Insulin receptor agonist
-
Development stage
Identification of candidate materials
-
Scheduled release date
2024
-
Partnership areas
Extended-release preparation technology, Protein stabilization technology
-
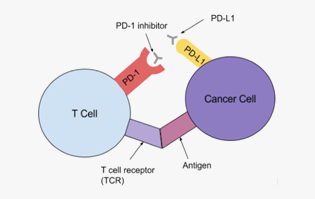
Indications
Solid cancer, etc.
-
Mechanism
Inhibition of the action of immunosuppressive signaling factors (immune checkpoint inhibitor)
-
Development stage
Identification of candidate materials
-
Scheduled release date
2027
-
Partnership areas
Effective lead antibody and antibody target against patients who do not respond to conventional PD-1/PD-L1 antibody, Antibody screening in-vitro evaluation system, Patient screening biomarker
-
Indications
Hypertension and dyslipidemia
-
Mechanism
Rosuvastatin (HMG-CoA reductase inhibitor), Olmesartan (angiotensin 2 receptor antagonist)
-
Development stage
Released in Korea
-
Scheduled release date
U.S./Europe in 2019
-
Partnership areas
Ideas for new combination preparation, Multiparticulate system (extended-release/enteric-coated pellets) technology
-
Indications
Endometriosis/myoma, prostate cancer, pre-menopausal breast cancer, and central orecicuiys puberty
-
Mechanism
Gonadotropin releasing hormone (gonadotrophin releasing hormone, GnRH) analog
-
Development stage
Released in Korea
-
Scheduled date
Approval in Japan/U.S. by 2020
-
Partnership areas
Partnership for U.S./Europe joint development and technology export
-
Indications
Prevention of nauseating·vomiting after administration of anticancer drugs
-
Mechanism
NK 1 receptor inhibition
-
Development stage
Manufacturing investigational drugs for overseas trials
-
Scheduled release date
Approval in Japan/U.S. by 2020
-
Partnership areas
Development of candidate drugs which require nanoparticle technology and products that can be applied to nano equipment, Partnership to enter into developed markets
-
Indication
Alzheimer’s dementia
-
Mechanism
Acetylcholinesterase inhibitor
-
Development stage
Manufacturing for overseas trials
-
Scheduled release date
L/O after Phase 1 clinical trial in 2019
-
Partnership areas
cGMP CMO cooperation for patches
-
Products of interest
UCDA/CA, choline alfoscerate
-
Development stage
Released
-
Partnership areas
- UDCA/CA : New manufacturing method of CA/CDCA using a variety of sources, new synthesis process for UDCA/CA
- Choline alfoscerate : New method of extraction/separation process
New platform technology
Daewoong Pharma is always opened to partnership in various fields.
Please take a look at the following areas for cooperation and propose your platform technologies.