FAQ & Suggestion
Daewoong Pharma is always waiting for creative research proposals.

FAQ
We have collected Frequently Asked Questions regarding O/C.
-
QWhat should I do if I want to open collaborate with Daewoong Pharma?
A1) Once you submit your idea/projects to our Website Suggestion, Daewoong Pharma will reach you within 7 days with initial feedback.
2) please contact us by email (opencollaboration@daewoong.co.kr) or phone (+82- 2-2190-6923) -
QWhat happens after the 1st feedback?
AAn in-depth review will be conducted after the CDA contract has been signed and the data has been provided at the additional process step. After passing the 2nd review, the contract will be concluded after negotiating the terms of the contract, and then the collaboration will proceed.
-
QWhat should I do if I want to discontinue the O/C with Daewoong Pharma? (Due to other good opportunities, personal problems, etc.)
AAccording to the contract concluded for O/C, we plan to set the collaboration phase/section gate in advance, make it possible to decide whether to maintain/stop collaborating at the gate point, and even if it is the gate position, you may terminate it if there are reasonable grounds for termination.
-
QIs it possible to use all of what Daewoong Pharma is providing for free?
AOnce the collaboration program with Daewoong Pharma is established, contracts are concluded according to the project/technology development stage, and Daewoong Pharma's core competencies listed on the website can be provided according to each cooperation model. At this time, what is provided by Daewoong Pharma is available under certain conditions. Daewoong Pharma will have the right to negotiate for the results of joint research and individual research, the terms of the contract will be negotiated according to factors such as contributions, development stages, and so on.
-
QWhat factors should be considered when selecting researcher-driven clinical research topics?
AYou should first consider whether the proposed project is novel and the scientific basis is valid through research materials such as papers and posters published previously. In particular, if the proposed ideas and research results are likely to find new therapeutic areas, it would be advantageous for selecting open collaboration project.
-
QI’d like to know the procedure of researcher-led research.
AAfter submitting the research plan, Daewoong Pharma will proceed as follows. Daewoong Pharma review → delivery of review comments to researchers (approved, supplemented, returned according to review comments) → completion of final examination → contract progress between Daewoong Pharma and researcher → start of research
-
QIs it possible to publish my research results for paper?
AAcademic presentation on the results obtained through the collaboration with Daewoong Pharma can be published in negotiation with each other.
-
QWhat should I do if I want to be involved in government projects?
AParticipation in government tasks is also determined by the official proposal and review process. Daewoong Pharma's R&D future direction and the current state of project resources will be considered and decision will be made according to the internal procedures. The proponent will be notified with the results.
-
QWhat are the benefits of research collaboration with Daewoong Pharma?
AWhen you become Daewoong Pharma’s partner, you can utilize the diverse core competencies of Daewoong Pharma. In addition, it’s possible to make various collaboration models such as joint research, technology introduction, research cost investment, JV establishment, etc. depending on the stage and level of technology, and the researcher’s compensation will be made accordingly.
-
QCan I propose technologies and materials outside of Daewoong Pharma’s interest areas?
AOf course. In addition to Daewoong Pharma's areas of interest, we also accept suggestions from various fields (pharmaceuticals, cosmetics, medical devices, etc.).
Review process
If you propose an idea, we will give you the 1st feedback within 1 week and then various types of consultation are possible.
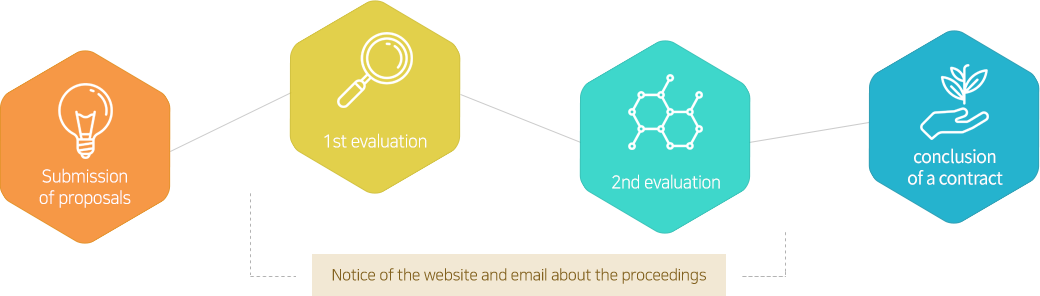
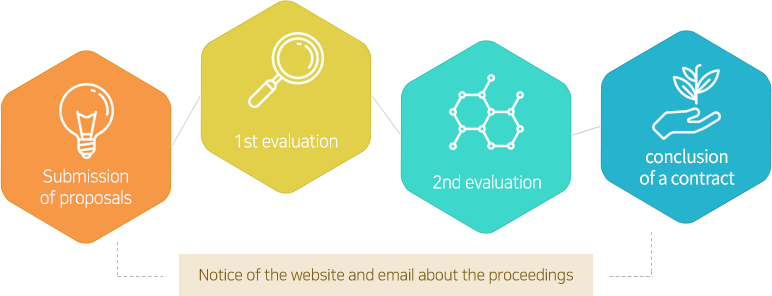
- step01 Idea Proposal
- Idea ProposalSubmit ideas in various forms regarding the purpose, project introduction, utilization plan, the collaboration model with Daewoong Pharma.
- step02 Review
- ReviewA dedicated team will review your submission based on the proposal, you can check the status of the submission, additional supplemental request, the decision of rejection within 1 week of submission.
- step03 Result notification
- Result notificationThe result of the review can be confirmed at the website and by personal mail.
- step04 Additional procedures
- Additional proceduresThere will be an additional negotiation regarding the conclusion of the research contract and the performance of the research.
Idea registration
Daewoong Pharma receives creative and challenging research projects in technology, clinical-related fields.
Application period
Year-round
Eligibility
Those who have more than 5 years of research experience after obtaining Ph.D and master’s degree from domestic and foreign research institutes.
Submission method and process
Daewoong Pharma is waiting creative partners to collaborate.
If you have any questions, please contact below.

person in charge
+82 2-2190-6923
opencollaboration@daewoong.co.kr