How we open collaborate
Daewoong Pharma provides a total solution for the success of your project through open collaboration.

Examples of successful collaboration
Daewoong Pharma focuses on win-win with our partners.
Daewoong Pharma has many good examples of open collaboration.
Provision of core competencies
Daewoong Pharma provides various solutions that will help lead
your ideas/projects to success through open collaboration.
Daewoong Pharma can provide Daewoong Pharma's clinical/pre-clinical candidate materials, libraries, compounds, and/or step-by-step stem cells. If you re-propose the previously made materials after incubation, we can discuss collaborative directions.
Laries
We can provide more than 100,000 in-house compounds to
study mechanism of action of new target and find effective materials
-
ㆍFragment library
(molecular weight: 350 or less, suitable for effective scaffold excavation, concentration: 10 to 50 mM)
-
ㆍIon channel-dedicated compounds library
(concentration: 10 mM)
-
ㆍKinase inhibitor dedicated library
(concentration: 10 mM)
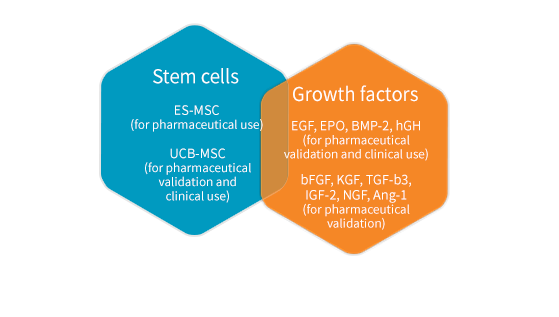
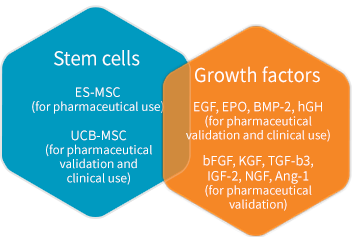
Stem cells
We can provide the stem cells and growth factors suitable according to the research stage.
Daewoong pharma has production facilities in the level of cGMP/EU-GMP level at home and abroad, based on skilled experience and know-how accumulated over 70 years from drug development to commercial manufacturing, provides CDMO(Contract Development and Manufacturing Organization) service.
Technology
Daewoong pharma which has a variety of specialized preparation such as depot injection, pills develops formulations optimized for customer needs.

Experience
Daewoong pharma provides CDMO solution based on skilled experience and know-how accumulated over 70 years

Quality-oriented
Daewoong pharma which considers quality first operates the facilities and quality management system in the level of cGMP/EU-GMP
Daewoong Pharma supports IIS (Investigator-initiated study) programs to discover new indications.
Support from Daewoong Pharma
Research fund required for clinical/nonclinical research
Review of research plan from Daewoong Pharma IIS Approval Committee
Support for clinical medicine and medical supplies(depending on the research subject)
Support for statistical processing (clinical trial plans and reports created) (disparity depending on the research subject)
Benefits to researchers
Broad financial support for various clinical areas
Advisory role of research-led clinical practice based on Daewoong’s clinical competence
Contribution of researchers’ performance as a result of clinical studies and further clinical improvement
Daewoong Pharma thinks the core of open collaboration is to form a network with the best experts at home and abroad and to do a virtuous circle to an organic relationship. It shares a good environment in which we can develop innovative medicines with global competitiveness utilizing various partnerships.
Daewoong Pharma runs ‘One stop POC center (Proof of Concept)’ in Daewoong Life Science Research Institute so that researchers who want to collaborate with us may verify their ideas.
DW-LAB
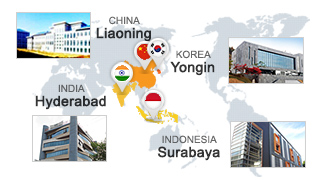
Domestic/foreign Daewoong Pharma Research Institute is opened to Daewoong Pharma’s collaborators. It’s located in Korea, China, India and Indonesia, and our partners can utilize state-of-the-art facilities.

Stem cell GMP
GMP facility dedicated to stem cells in Daewoong Pharma Bio Research is to produce a stem cell clinical sample and products built on the needs of researchers in the field of regenerative medicine, which is open space for Daewoong Pharma’s partners.

Smart Office
Daewoong Pharma Bio Center implemented smart office environment to enable researchers actively to exchange by applying smart office concept, and collaboration targets can use in conjunction with DW Lab.
Partnership compensation system
- step01 Idea
-
Standard
- ㆍNew product/technology development concept and document of proof
- ㆍRequired : Scientific feasibility
- ㆍOptions : Marketability, Feasibility
Procedures
- 1. 1st rapid review > Confidentiality Agreement(CDA)
- 2. 2nd review > Feasibility test
- 3. Joint research/Introduction contract
Performance allocation
- ㆍFeasibility expenses : Covered by Daewoong Pharma
- ㆍJoint research expenses : Negotiation
- ㆍPatent rights : Joint
- ㆍExclusive license : Daewoong Pharma royalty fee
- step02 Search
-
Standard
- ㆍPossession of efficacy by realizing the concept, POC, basic toxicity data
Procedures
- 1. 1st rapid review > Confidentiality Agreement(CDA)
- 2. 2nd review > Conclusion of MTA > Feasibility test and Due Diligence
- 3. Joint research/Introduction contract
Performance allocation
- ㆍMTA evaluation : Covered by Daewoong Pharma
- ㆍJoint research expenses : Negotiation
- ㆍPatent rights : Joint
- ㆍExclusive license : Daewoong Pharma royalty fee
- step03 Development Early stage
-
Standard
- ㆍNonclinical toxicity ~ clinical phase 1
Procedures
- 1. 1st rapid review > Confidentiality Agreement(CDA) > Technology/product presentation
- 2. 2nd review > MTA or Due Diligence
- 3. Introduction or conclusion of joint research agreement
Performance allocation
- ㆍDevelopment cost : Mutual Negotiation
- ㆍExclusive license : Daewoong Pharma(territory negotiation) royalty fee
- step04 Development Last stage
-
Standard
- ㆍClinical phase 2 ~ 3
Procedures
- 1. 1st rapid review > Confidentiality Agreement(CDA) > Technology/product presentation
- 2. 2nd review > MTA or Due Diligence
- 3. Introduction and conclusion of joint research
Performance allocation
- ㆍDevelopment cost : Mutual Negotiation
- ㆍExclusive license : Daewoong Pharma(territory negotiation) royalty fee
- step05 Commercialization
-
Standard
- ㆍPermission submission preparation stage
Procedures
- 1. 1st rapid review > Confidentiality Agreement(CDA) > Technology/product presentation
- 2. 2nd review > MTA or Due Diligence
- 3. Introduction and conclusion of joint research
Performance allocation
- ㆍDevelopment cost : Mutual Negotiation
- ㆍExclusive license : Daewoong Pharma(territory negotiation) royalty fee